Table of Contents
Types of Batteries
- In this article, we’ll discuss all types of batteries we are using in our projects. Types and explanation are gathered from the resources on the internet and practice experiments.
- Detailed description and their advantages and disadvantages are explained in each battery type further. Also, images are attached for more reference.
- Datasheet for each battery type as example are give also the buy link for each battery is provided. so, there is first type of battery
Lead-Acid

What is lead-acid battery?
- These are the most famous and very cheap available battery available. The popularity due it’s popularity is due to its large number of usage and sizes available.
- Although they are bulky in sizes but yet they are popular for their capability of delivering high current to devices. Along with this, their low for more power ratings make them ideal for low cost projects or low budget devices.
- Apart from this, these are most recycled battery type among all other type of batteries. But they also have some issues which are its drawback.
lead acid battery Construction:
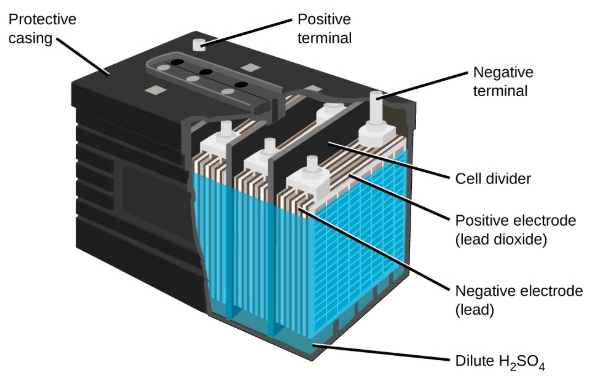
- These lead acid batteries come in many types, some popular ones are, SLA, MF, AGM, and VRLA. We’ll discuss them all in common, as their construction is quite similar.
- These batteries mostly comprise Electrodes, Lead plates, and an electrolyte which are the basic composition of a Lead-acid battery. For different types fo batteries, some other materials or things are used which are discussed further.
- Between the positive and negative electrodes, there are separators that allow ions to flow and hence complete the circuit of battery composition.
- In AGM type, the separators in replaced with glass fiber mat soaked in electrolyte. This increases the exchange or passing of gasses produced during the charging and discharging process.
- For this purpose, the electrolyte in AGM is replaced from liquid to semi saturated type. While electrolyte in the most basic construction of the lead-acid battery is a mixture of sulfuric acid and water(Distilled).
- In VRLA batteries, which are basically sealed batteries, vents are provided for the release of gases produced inside the battery.
- These (VRLA) batteries are also called gel batteries, we have seen these most commonly in household devices like insect rackets.
- Due to the gel-type electrolyte, the advantages of AGM and VRLA are the same. These all make them mostly used batteries in extreme conditions, as they have low freezing and high boiling points than basic (wet) or AGM types.
- These all advantages of the AGM and VRLA make them maintenance-free as they do not require watering and gas valve for gas blow off.
Lead Acid Battery Charging and Discharging:
- The charging and discharging of the lead-acid batteries can be written in the chemical equation for more reference. These are given below.
- During discharging the dissolves sulphuric acid is converted into the water, as the positive and negative terminals (both PbSO4).
- Pb(s) + HSO−4(aq) → PbSO4(s) + H+(aq) + 2e− this the negative plate reaction, PbO2(s) + HSO−4(aq) + 3H+(aq) + 2e− → PbSO4(s) + 2H2O(l), this is the positive plate reaction.
- The overall reaction can be written as Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l).
- The total energy released is 2F (Faraday) which is Coulomb is 1,92,971 per mole of lead resulting in, the formation of 2 moles of water.
Applications of lead acid battery :

- There are many uses of the lead-acid battery, we discuss some of its applications here. These are used from small devices like an insect racket to big and heavy machinery like a forklift.
- All types of automobiles use the lead-acid battery either SLA or VRLA for ignition of engine and electric uses. Also, these batteries need to be recharged with both CC & CV techniques for a better life cycle.
- Most of the electric toys used lead-acid batteries also in the robotics field we use the lead-acid battery for most of the low-cost projects.
- These are also used in heavy machinery like Forklift in factories and industries which require drawing a large amount of current in a very short time.
- And many more types of equipment which need drawing large current and power in a very short time.
Advantagesof lead acid battery:
- The main and most important advantage of the lead-acid battery is the cost over the other types of batteries. If we calculate the price of a lead-acid battery in terms of watt/per hour, is rather very cheap and cost-effective in all types of batteries.
- Secondly, the construction and packaging of the lead-acid battery are tough & rigid which overall all increases its durability over other batteries
- Also, these batteries can be recharged, and mostly the type of which is used in homes or UPS can easily be used as they require only water refilling as maintenance.
- Further, these batteries have the capability of delivering large current to load and appliances, hence these are more popular among high power devices and tools.
- Also, if the battery is overcharged or discharged the gasses can easily escape either through the gas valve or the water opening timely despite other batteries which leak or get puffed up.
Disadvantages:
- Most importantly, these batteries type has the lowest energy density which makes them non-ideal for portable and mobile devices or in simple words handy devices.
- The electrolyte is dangerous and quite risky while transporting these batteries, a these may leak or spill in between.
- Another disadvantage of these types is that you cannot use them just after the charging or just after water refilling charging as you need to wait for 12-14 hrs for voltage stabilization.
- Although they are the most recyclable battery type, the material used in these LEAD(Pb) is toxic which can cause harm if improperly recycle.
Ni-Cd
Now the question is what is a Nickel-cadmium battery and how do they work?

- These are the most common portable batteries in all types of batteries you may seem around in various devices, right from RC Toys to various household devices.
- These batteries are similar to other cell or cylindrical type batteries, but the construction and effects are different from others.
- Unlike Lead-Acid batteries, they come in a cylindrical package and a nominal voltage of around 1.2V to 1.4V. These need to be connected in series and parallel for making the appropriate battery pack for the power supply.
- But they also have some characteristics similar to Lead-Acid battery, like they can deliver high current at their full capacity. This even doesn’t affect their life and performance cycle.
- Along with this they can adapt to fast and easy charging even if you charge them after a long time. But in the recommendation, they must be taken into use in tasks that require periodic usage, or due to their self-power loss, they may discharge overtime and get damaged.
NI-CL Battery Construction:
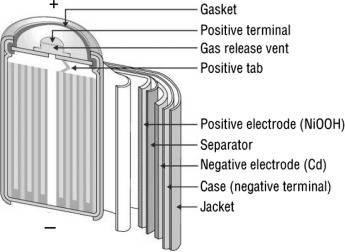
- The construction of these batteries is rather quite compact as compared to a lead-acid battery. They come in two types or sizes which are mostly used, AA size and D size, but the construction for both is the same.
- The batteries are enclosed, or they have metal packaging with a self-sealing plate, including a self-sealing safety valve at the positive terminal.
- The positive and negative terminal electrodes are separated from each other by a separator, but both electrodes are rolled in the form of a spiral in the metal casing.
- The electrolyte is of some alkali solution, which separates both positive and negative electrode.
- The positive electrode is made up of NiO(OH) and the negative electrode is of Cd. These both are rolled up as stated above with electrolyte in between them as medium for passing the ions, with the separator sandwiched between both the electrode layers.
ni-cl battery Charging & Discharging:
- The charging and discharging of the Ni-Cd batteries can be shown in form of a chemical equation as below. The equation for both positive (Ni(OH)2) & negative (Cd(OH)2) electrodes is for the discharge process.
- Cd + 2OH– → Cd(OH)2 + 2e–. This is for the Cd or positive electrode.
- 2NiO(OH) + 2H2O + 2e– → 2Ni(OH)2 + 2OH–. This is for the Nickel Oxide or negative electrode.
- The overall equation of the reaction is 2NiO(OH) + Cd + 2H2O → 2Ni(OH)2 + Cd(OH)2.
- The reaction during the discharge process is as above, i.e., from left to right, but for the recharge process, it is reversed from right to left unlike the lead-acid battery.
Applications of nickel cadmium battery :
- The main or most common application of the Ni-Cd battery is forming battery packs of the desired value by arranging them into series and parallel.
- Single cells are used in toys and household devices like RC toys and electric trimmers.
- The smaller button cell construction of these types of batteries is also used in handheld devices or in BIOS memory backup batteries in computers.
- Also, with growing technology we can also see these batteries used in vehicles as battery packs nowadays as these can provide large current as a lead-acid batteries without affecting their capacity or battery life.
Advantages:
- First of all they can adapt to fast, quick and easy charging with any balanced charger available or them. Also, theses doesn’t affect their life cycle or capacity, even using after along period of time.
- Secondly, they have high energy density as compared to Lead acid battery. The AA and D Size battery packages can offer a same amount of power as a Lead-Acid battery, but in a smaller package or space.
- Even though the material used in the construction is not as durable and strong as Lead-Acid battery, yet they are quite durable and robust.
- They are also recyclable through thermal treatment under vacuum to recollect the Cd. Ni is also recycled in the form of Ni-Fe alloy.
- If the battery is overcharged then the excess water above the limit of safety valve, which is formed during the process, it released in vapour state.
Disadvantages:
- The most important and harmful disadvantage of these batteries is that they are formed or the composition of the electrodes is of toxic materials. Which is discharged in the environment during the recycle or and other ways can be harmful to the environment.
- Secondly, if the battery is overcharged then the excess water will be released from the safety valve, but it will affect its capacity.
- This type of battery is also prone to memory effect, which is caused by the same charging and discharging cycles of the battery regularly.
- These batteries also self discharge at a rate of 20% per month under identical conditions.
Ni-MH

- Now let’s discuss another type of battery NI- MH similar to Ni-Cd i.e., Ni-MH which stands for Nickel-Metal Hydride Batteries.
- These batteries are more popular than Ni-Cd due to 3-4 times more capacity, which overall increases their energy density of them.
- The sixes and packages of the Ni-MH batteries are similar to the Ni-Cd batteries, but the current rating is much more than Ni-Cd batteries
- These are identical to alkaline batteries and even can be used as their replacement, the only issue is of the slightly less voltage.
- the full name of NI-MH battery is nickel metal hydrite battery
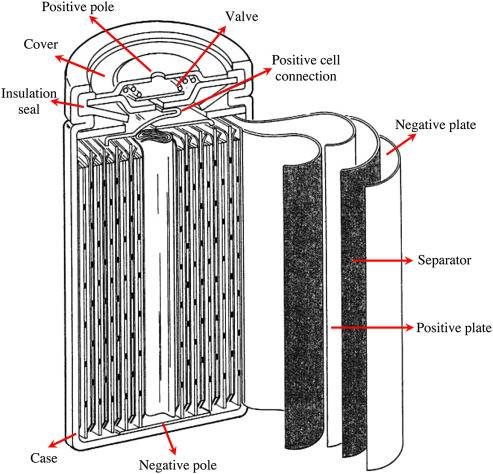
- The construction of the Ni-MH batteries are similar to the Ni-Cd batteries, but they are both different in the material and separators used.
- The positive electrode is made of the same material as Ni-Cd, or NiO(OH), while the negative electrode is made of the Hydrogen absorbing alloy instead of Cadmium.
- The electrolyte, in this case, is Potassium Hydroxide (KOH) which is also filled in between both electrodes which are rolled up in the form of a spiral as a Ni-Cd battery separated by a separator.
- These are also capable of delivering high current as similar to Ni-Cd, which is an advantage of them over alkaline batteries in single charge use.
- Furthermore, these also have the self-sealing safety valve for the release of gasses during the overcharge process, like in case of Ni-Cd batteries construction.
nickel metal hydrite battery Charging & Discharging:
- Like other batteries, the charging and discharging process of the Ni-MH batteries can be expressed in terms of chemical equations.
- But unlike Ni-Cd batteries, the reaction in these batteries is reversible to indicate by the reversible arrow ⇄, which indicated that the process works on the principle of equilibrium.
- H2O + M + e− ⇌ OH− + MH. This is the negative electrode reaction, which is built of Metal Hydride.
- Ni(OH)2 + OH− ⇌ NiO(OH) + H2O + e−. This is the equation of the positive electrode, i.e., of NiO(OH).
- The reaction proceeds left to right during the discharging process and right to left during the charging process, as same in Ni-Cd batteries.
- The separator used in these batteries is of Hydrophilic polyolefin nonwovens are used for separation between both the electrodes, rolled in the form of a spiral
Applications of NI-MH battery :

- It’s most of the applications are similar to the Ni-Cd batteries. Due to its more popular AA and D-size and large current rating, it is commonly found in various battery packs.
- In RC Toys and consumer electronics used in a house, also in power tools like electric drills and cutter due to their large current supplying capability.
- It is also used in Vehicles as battery packs instead of Lead-Acid batteries or in electric vehicles as an alternative to Li-Ion batteries which are used conventionally.
- Despite these, it is also used in older laptops in place of Li-Ion and in cell phone as a portable power source with higher power capacity.
Advantages:
- The main advantage of these batteries is the more capacity than the Ni-Cd batteries, which is great in terms of energy density.
- Secondly, the material used in manufacturing the batteries is not as toxic as Ni-Cd, so it is more environmentally friendly than Ni-Cd ones.
- There are many ways of charging these batteries like either monitoring changing voltage or temperature, or you can also use trickle charging method.
- In changing voltage or temperature techniques the voltage or temperature changes are being monitored over time and according to the datasheet of the battery the current of C value is set.
- In the trickle charging method, the battery is charged constantly at 0.1C current. But this method is for a long time and if overcharged can reduce battery life.
- For safety features, it has a bimetallic resettable fuse that opens if either the current or the temperature is too high and closes again when it is under a suitable range.
- They also have a relatively low self-discharge rate, which is also an advantage of using them over Ni-Cd batteries.
Disadvantages:
- The main disadvantage of these batteries is that they have a low life cycle, also after a few hundred charges you can witness the drop in their capacity.
- Secondly, if you over-discharge these batteries then these may also show reverse polarity which can permanently damage the batteries.
- Also, it is advised either to use appropriate power battery packs for power tools or if you used underrated power battery packs then the life cycle of individuals may shorten.
- Due to it having high energy density than Ni-Cd ones, these also have a high cost than those, which can be a bit costly for large scale.
- It is recommended to use desired battery balanced charges for charging the batteries, or it may damage the batteries permanently due to more complex algorithm charging than Ni-Cd.
Li-Po
the most common use battery available in all types of batteries. it is used in many day-to-day applications. now are want to know i.e what is lipo battery.

- This is one of the most famous, mostly used batteries in projects. Due to its high capacity and wide range of sizes and availability.
- Li-Po or Lithium-ion Polymer battery is another type of battery with polymer electrolyte instead of conventional Liquid or semi-liquid electrode.
- These batteries work on the principal of intercalation and de-intercalation between positive and negative lithium electrodes.
- These batteries are rather very cheap as compared to the Ni-Cd and Ni-MH cells also they come in thin to thick sizes which make them ideal for using in small spaces.
lipo battery Construction:
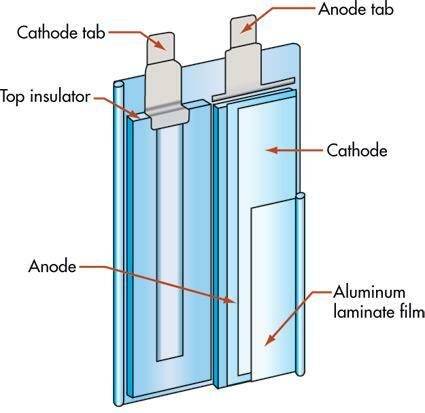
- The construction of the Li-Po is not spiral as in the case of Ni-Cd and Ni-MH, but both electrodes are individually wrapped but both the electrodes are of lithium only.
- For separating both the electrodes, a separator of material like polythene or polypropylene is used, which is microporous and allows the ions to exchange.
- The positive electrode is usually a mixture of 3 parts that are lithium with transition metal oxide, a conductive additive, and a poly binder.
- The negative electrode is similar to the positive electrode i.e., the mixture of 3 parts the only difference is that there is a mixture of carbon with lithium.
- The electrolyte is a polymer as stated above instead of conventional liquid or semi-liquid electrolyte, but this doesn’t affect the capacity or life.
- The outer covering or the pouch in which the battery is packed is la layer of aluminium foil sandwiched between two polymers.
lipo battery Charging & Discharging:
- Like other batteries, the equation of charging and discharging cannot be written, I’ll explain the full working and all charging and discharging issues.
- Like stated above, it works on the principle of intercalation of both electrodes. The nominal or common voltage of a single cell is 3.7V.
- The voltage ranges from 2.7V to 4.2V, where 2.7V-3.0V is the full discharge state and 4.2V is the fully charged state, this is for a Lithium metal oxide battery.
- For Lithium Iron Phosphate batteries, the charged voltage range is 3.6V -3.8V, and 1.8V-2.0V is discharged state range.
Applications of lipo battery:
- These batteries have very high and most demanding usages. Due to their various size and capacity options, they are 1st choice for any project.
- They are used in most RC flying toys, as they require lightweight and high-capacity batteries with high current ratings.
- Nowadays, these batteries are also used in various household and handheld devices due to their compactness and less spacing-taking capabilities.
- Also used in electric vehicles as a replacement of the Li-Ion, Ni-Cd & Ni-MH cells as these are quite costly and require a decent and fixed amount of space per cell.
- Moreover also used in UPS and jump starters as a combination of cells, as the combination can supply large current in emergency situations.
Advantages:
- The main advantage of these batteries is the shape and sizes of the batter and the high energy as compared to Ni-Cd and Ni-MH batteries if compared on the same weight and volume bases.
- The wide range of choices and C rating along with S&P battery packs are a big advantage over other battery types.
- Along with these, batteries have low internal resistance which allows them to deliver high current during required times such as RC toys.
- They have higher energy density than that of Ni-Cd and Ni-MH batteries, which are costly. These batteries can over more amount of power at the same cost as that of a cell of Ni-Cd or Ni-MH ones.
- The terminals of these batteries are easily soldered unlike cell packaging of any other battery as those require either a Spot Welder or some sandpaper rubbing and then soldering.
Disadvantages:
- The main disadvantage of these batteries is that they puffed up are kept full charge or sometimes also leak, leaving a foul smell around them.
- These batteries need s to be charged at CC/CV methods or the cell may damage over time or lose its capacity.
- Also, if you short circuit the battery by chance, the battery may cause fire and or may explode in certain situations.
- If these batteries are used at low temperature like below 10 °C then you’ll see a degradation in their performance and capacity, same as for high temperature like above 50 °C these batteries have a high chance of exploding.
- The terminals, if soldered without any heat sink or use of thick wires the point or terminals may tear off, and you may damage your battery.
- High capacity battery needs constant a CC/CV charger and a battery monitor with corresponded to each cell especially for drones and RC planes.
Li-Ion

- This is the last type of battery we are going to explain what is li-ion batterythe , these are very demanding and common among hobbyists for use in almost every project.
- These batteries are similar to Li-Po batteries, but the only difference is in the construction and working of these batteries, which make them very ideal for almost every project.
- The shape and sizes of these batteries are usually AA or AAA sizes, but also they are custom-made in various sizes on demand, like as found in mobile phones.
- These batteries have the highest energy density among all batteries and are relatively costlier than any other type, but like coin has two sides, these have advantages also.
- The nominal or normal voltage of any battery size of Li-Ion battery is 3.7V and if you charge the battery you need to follow the CC/CV methods for each cell to ensure their battery life.
the lipo battery Construction:
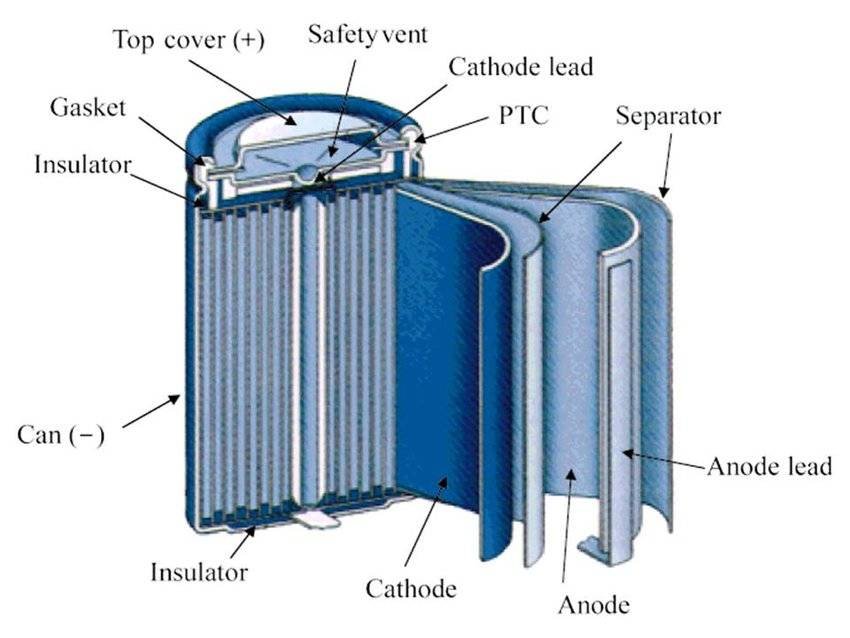
- As these batteries are commonly found in either AA or AAA size, we’ll discuss the construction of bases on these two. Also, in some places, we’ll give references for other sizes.
- The positive electrode of these batteries is mostly made of Metal Oxide, which can be of one of these 3 materials. A layered oxide such as lithium cobalt oxide, polyanion such as Lithium Iron Phosphate, or a spinel such as lithium manganese oxide.
- The negative electrode is made of carbon, mostly graphite, which in its fully lithiated state of LiC6 has a capacity of about 372mAh/g.
- The electrolyte in these batteries is a lithium salt in an organic solvent, as for the separator between the electrodes it is basically polyethylene or polypropylene.
- The basic outer covering that is found in cells is of metals case without bulged surface as in normal batteries, whereas large cells have threaded terminals for screwing the wires and connectors.
lopo battery Charging and Discharging:
- The charging and discharging reaction of Li-Ion batteries can be denoted through the equation which is given below. Also, the methods and consequences of charging and discharging are discussed below.
- CoO2 + Li+ + e– ⇌ LiCoO2, this is the positive half equation which denotes Lithium doped with cobalt oxide substrate.
- LiC6 ⇌ C6 + Li+ + e–, this is the negative half electrode of the lithium with graphite(carbon).
- The overall equation of the reaction is LiC6 + CoO2 ⇌ C6 + LiCoO2, this is a reversible equation that denotes discharging from left to tight and charging from right to left.
- Over discharging the batteries lead to the supersaturation of Lithium Cobalt Oxide which leads to the formation of lithium oxide, which is an irreversible reaction. Li+ + e– + LiCoO2 → Li2O + CoO.
- Overcharging the batteries is also destructive for them as overcharging leads to the synthesis of Cobalt oxide, which as if the battery is kept in charge state make them puffed up.
Applications of lipo battey:
- These are mostly used and very popular battery, and it has numerous application and uses. The highest energy density and cost-to-energy ratio make them ideal for usage.
- The most common use which everyone has is the mobile phones. Modern smartphones require a large power capacity battery but less weight, a Li-Ion battery is ideal for these.
- Secondly, modern laptops like MacBooks and book-type laptops and tabs are also the major field of application of these batteries.
- Power tools and Hand-held devices which are used in houses are also very common uses of Li-Ion batteries,
- Wireless devices and automobiles are also a growing field of usage of the Li-Ion batteries. The cell, of AA and AAA sizes, are most common.
Advantages:
- The first advantage which makes it ideal is its high energy density, which outperforms every battery type in many comparisons.
- Secondly, the various sizes and low cost of producing the custom size battery make it easy to afford batteries for low budgets projects.
- They can be easily recycled also can be reused more easily than any other batteries like Lead-Acid and Ni-Cd or Ni-MH, which are either harmful to the environment or hard to recycle.
- They have a very low self-discharge rate, like 2% to 3% per month of the original C rating of the battery. Also, the adequate rate of temperature range makes them able to use in almost all conditions (5 °C to 45 °C).
- Though the charging methods are the same as CC/CV, the charges are easy to afford same in the case of Li-Po battery but these both need different charges as per their type.
- This battery is almost free from memory effect, which is the most important issue in cases of Drones and RC toys and devices.
Disadvantages:
- The main disadvantage of these batteries is they need care and monitoring while charging as higher temperature during charging may lead to leaking or even burning of battery causing a fire.
- The terminal of these batteries in AA or AAA size batteries needs to be either spot welded or first rubbed with sandpaper and then soldered as same in the case of Li-Po batteries.
- You cannot keep the battery in the charged state as it will make the battery puffed up and lead to the destruction of the battery. Even troubleshooting methods on YouTube didn’t work as it ultimately result in the loss of the capacity of the battery.
- They are not as good as Ni-Cd or Ni-MH in power tools which are portable as discharging current is less than compared to other both types.
- You cannot fold or put excessive pressure on rectangular type packages as it may result in a leak or immediate fire, same as for Li-Po batteries.